Important Information Regarding Our Voluntary Recall
To the valued members of our healthcare community: In response to a broader FDA investigation of infant botulism, all ByHeart Whole Nutrition Infant Formula cans and Anywhere Packs™ nationwide have been recalled. This action is being taken in cooperation with the U.S. Food and Drug Administration (FDA) as we continue to investigate every facet of our process – from ingredient-sourcing to our manufacturing process and facilities, packaging, and transportation.
We urge parents and caregivers to stop using ByHeart formula immediately, monitor their child for symptoms of infant botulism, and seek medical care immediately if symptoms develop. Healthcare providers should discard all in-office samples.
The safety and wellbeing of every infant remains our top priority. For the most current information, FAQs, and contact information, please visit the link below.
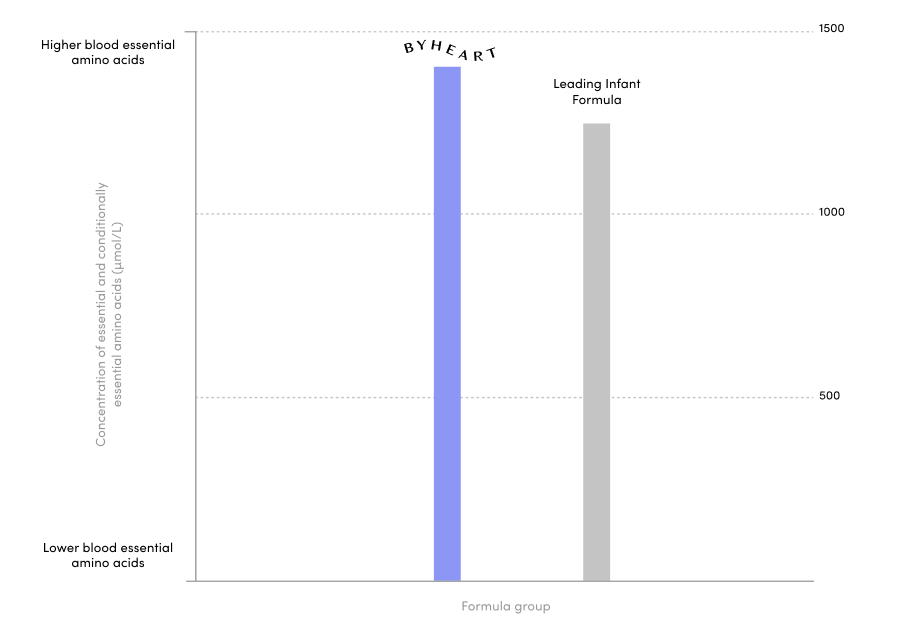
A clinical trial 25 years in the making.
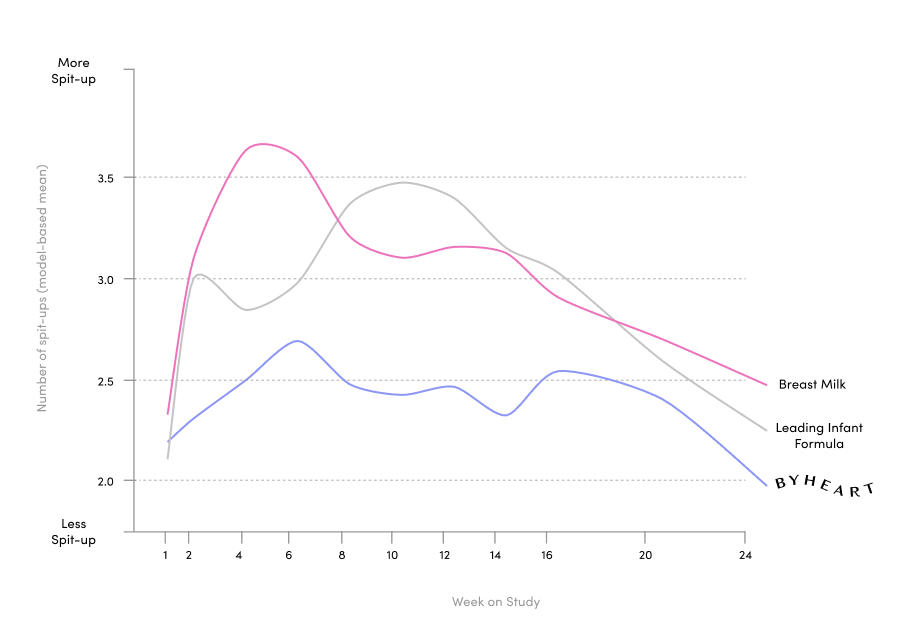
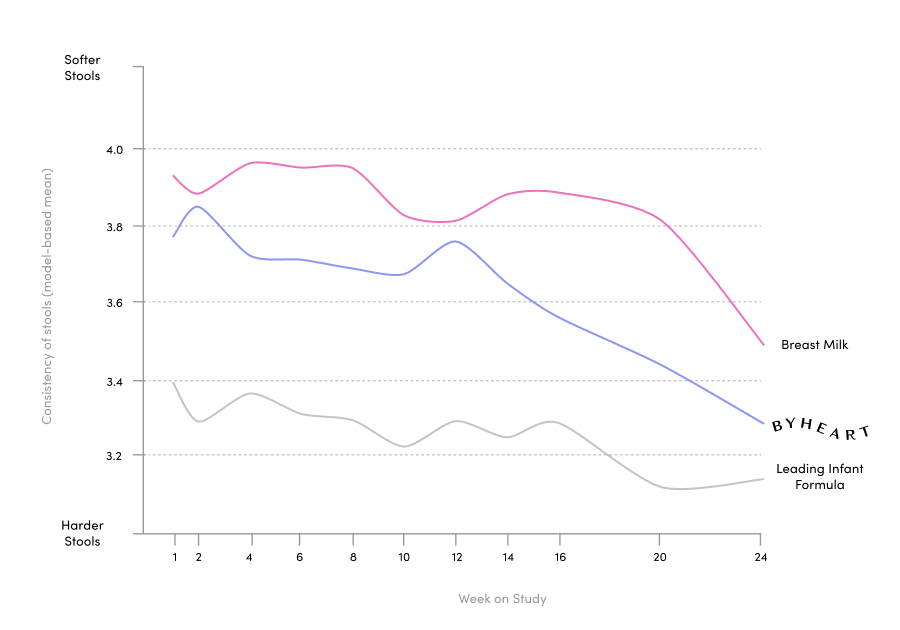
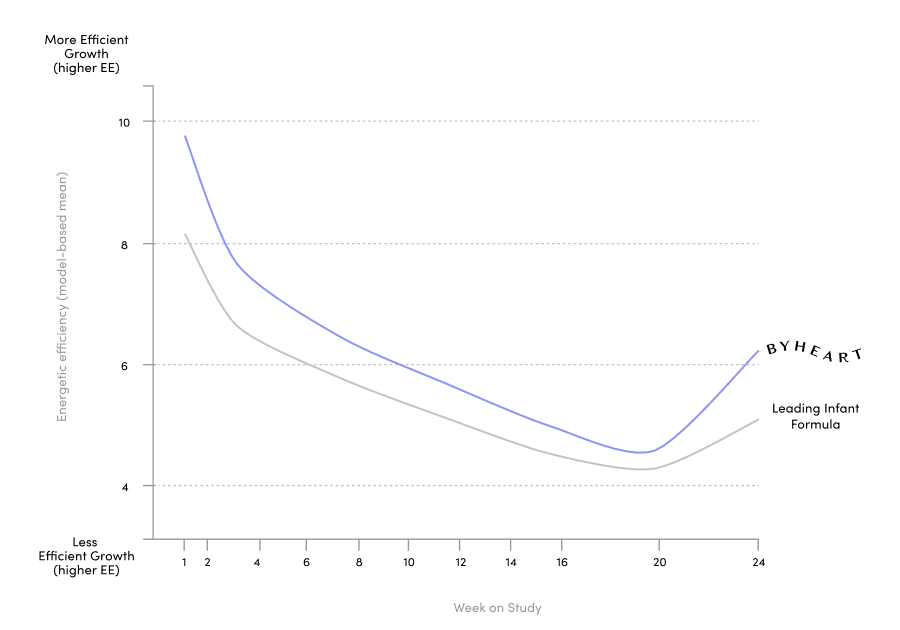
ByHeart ran one of the largest clinical trials for an infant formula brand in the US in 25 years, published in the Journal of Pediatric Gastroenterology and Nutrition. We included 311 healthy, term infants and assigned them to either ByHeart formula, a leading commercial formula, or a human milk-fed reference group. We were also the first new brand to add a comparison to human milk.
Our 6-month randomized, double blind, multi-center trial exceeded FDA requirements with a systems biology approach.